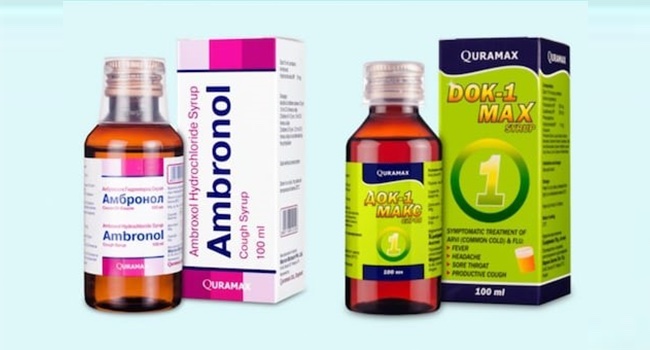
Avoid AMBRONOL and DOK-1 MAX Syrup.
The public has been warned by the National Agency for Food and Drug Administration and Control (NAFDAC) not to use or buy two contaminated products called AMBRONOL syrup and DOK-1 Max syrup.
The World Health Organization was informed about the two medical products in Uzbekistan, a country in Central Asia, according to a statement from NAFDAC (WHO).
According to the agency, laboratory testing revealed that both products had unacceptable levels of diethylene glycol and/or ethylene glycol as contaminants, which is why they failed to meet quality standards.
UN issues warnings about severe malnutrition in Nigeria and 14 other nations
Diethylene glycol and ethylene glycol, which are present in these products, are toxic to humans and can have toxic and fatal effects, claims NAFDAC.
It was noted that the toxic effects could include acute kidney injury, which may cause death, as well as headaches, nausea, vomiting, diarrhea, and an inability to pass urine.
However, NAFDAC urged anyone in possession of the subpar product to stop using, selling, and submitting stock to its nearest office.
Before using liquid dosage forms in medicines, the agency advised Nigerian manufacturers to check for contaminants like ethylene glycol and diethylene glycol.
“Importers, distributors, retailers, and consumers are advised to exercise caution and vigilance within the supply chain to avoid the importation, distribution, sale, and use of the substandard (contaminated) syrups, even though the products are not in the NAFDAC database.
All medical supplies must be purchased from licensed and authorized vendors. It is important to carefully inspect the products’ physical integrity and authenticity, according to NAFDAC.
Please refrain from using these inferior products if you have them. You are urged to seek immediate medical advice from a licensed healthcare provider if you or someone you know used these products or experienced any negative side effects or event after use. The use of these products may cause adverse events or side effects, which should be reported to the nearby NAFDAC office by consumers and healthcare professionals